Abstract
Background: Acute myeloid leukemia (AML) is a genetically heterogeneous clonal disease with highest incidence in the elderly. Advancing age and complex karyotype aberrations, two strong adverse prognostic factors in AML, are closely interconnected and responsible for poor treatment outcome. Chromosomal instability (CIN) is a major contributor to genetic heterogeneity and clonal diversification in AML and its level increases with advancing age both in normal and malignant cells. In elderly healthy individuals clonal mosaicism for chromosomal aberrations in blood cells is associated with an increased risk of a subsequent hematological malignancy. Despite major advances in understanding the genetic landscape of AML and its impact on disease pathophysiology, why genomic integrity declines with age is barely uncovered. For accurate chromosome segregation, sister chromatids are tightly associated until the spindle assembly checkpoint is satisfied, allowing for anaphase‐promoting complex (APC/C)‐mediated anaphase onset. Sister chromatid cohesion is established by the ring‐shaped cohesin complex consisting of four subunits (SMC1A, SMC3, Rad21, and either SA‐1 or SA‐2). Age‐associated CIN in human oocytes seems to be the consequence of cohesin decay with increasing maternal age.
Aims and methods: Based on frequent loss‐of‐function mutations in cohesin complex components in human malignancies including AML, we sought to investigate whether decreased cohesin subunit protein levels are associated with aging and leukemogenesis in human individuals by analyzing mononuclear cells (MNCs) from 95 healthy blood donors and 48 AML patients.
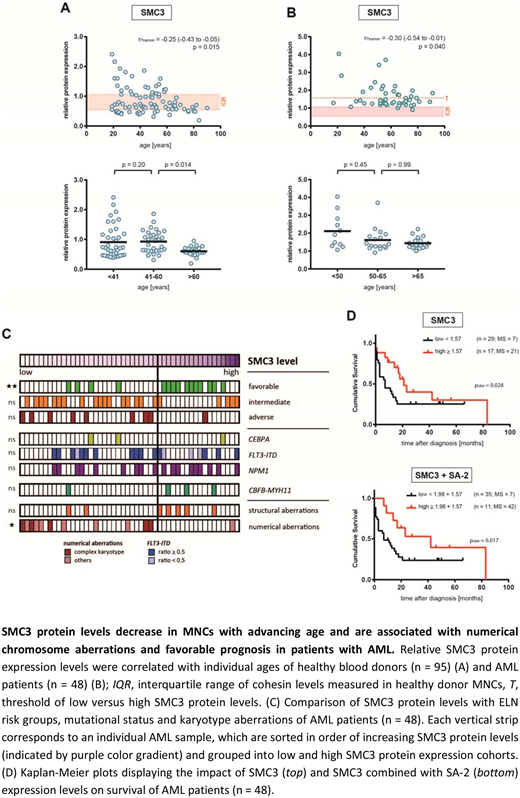
Results: Western Blot analysis of peripheral blood MNCs from 95 healthy individuals with a median age of 48.5 years (range 19-90) revealed that SMC3 and SMC1A cohesin subunit levels were significantly reduced with advancing age (Fig. 1A). Interestingly, SMC3 protein levels were most prominently reduced in individuals older than 60 years. For SA-1, a borderline significant age-associated decline in protein expression was observed. Surprisingly, compared to healthy donors, Western blot analysis of MNCs from peripheral blood (n=) or bone marrow (n=) from 48 AML patients (median age: 59 years, range 17-91) showed significantly elevated protein expression levels of all cohesin complex components (Fig. 1B). Nevertheless, SMC3 protein levels significantly declined with advancing age in AML patients, similar to the situation in healthy individuals. High SMC3 protein levels were significantly associated with ELN favorable risk group classification (low versus high protein levels, p=0.0010) (Fig. 1C). Moreover, the occurrence of numerical chromosome abnormalities and complex karyotypes increased with decreasing SMC3 protein levels (low versus high protein levels, p=0.0185). The association between age and SMC3 protein levels remained significant after adjusting for karyotype and risk group in multivariate linear regression analysis with age (p=0.03), risk group and numerical karyotype aberrations being independently associated with SMC3 expression (adverse versus favorable risk, p=0.027; numerical aberrations versus normal karyotype, p=0.048). No correlation of SMC3 levels with the mutational status of NPM1 and FLT3 or the CBFB‐MYH11 fusion nor an association of other cohesin subunit expression levels with any of the parameters analyzed was found. Compared to AML patients with lower SMC3 expression, patients with higher levels had a significantly longer overall survival. No survival differences between patients with low versus high expression levels of other cohesin subunits were found. Interestingly, patients with elevated levels of both SMC3 and SA-2 experienced the longest overall survival.
Conclusions: In summary, SMC3 cohesin subunit protein levels decline with advancing age in both healthy individuals and AML patients. Reduced SMC3 levels are associated with numerical chromosome abnormalities, complex karyotypes and outcome in AML. These findings are remarkable as overall cohesin mutations are neither associated with karyotype aberrations nor with an impact on survival and possibly explained by the fact that with the exception of SMC3 for all of the other cohesin subunits at least two versions exist, which ‐ at least partially ‐ complement each other.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.